More parents STANDING UP against coronavirus vaccines targeting children
05/14/2021 / By Ramon Tomey
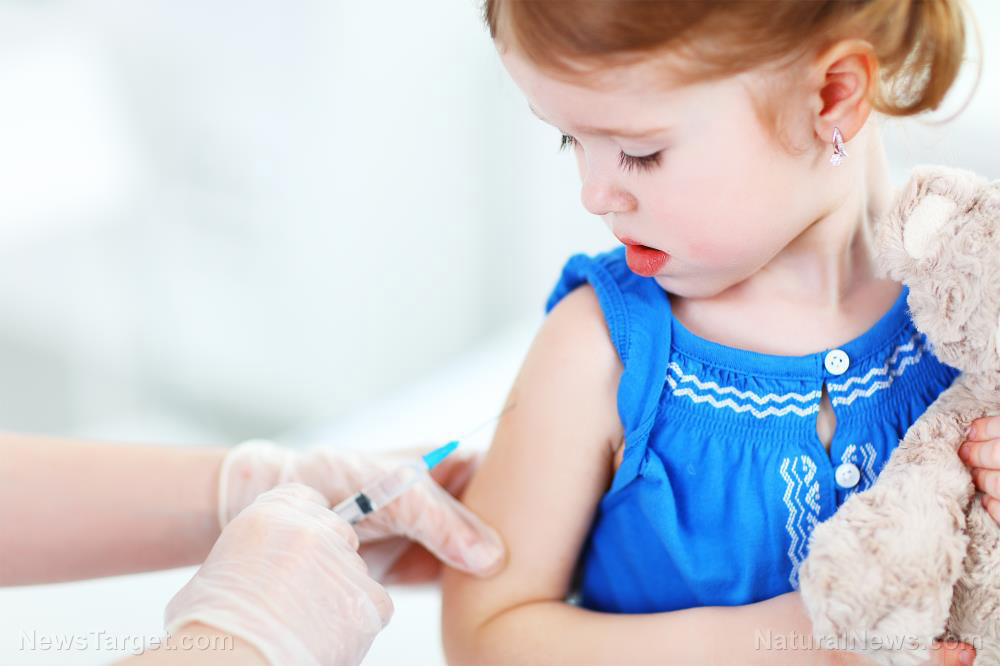
A survey found that more parents have expressed opposition to their children getting Wuhan coronavirus (COVID-19) vaccines. The sentiment followed drug manufacturer Pfizer’s application for emergency use authorization (EUA) to use its vaccine for younger patients, which would allow it to be used to inoculate children from two years old to 11 years old.
The survey, published in the April 2021 edition of the Kaiser Family Foundation’s (KFF) Vaccine Monitor, found that 19 percent of respondents would not get their child immunized. It also found that 32 percent of parents preferred to “wait and see” how the COVID-19 vaccines work before having their child immunized. Meanwhile, 15 percent said they would only have their child vaccinated against the Wuhan coronavirus if schools require it.
The survey also showed that vaccine hesitancy is strong among Black and White parents. Twenty-nine percent of Black respondents said they would not allow their children to get the COVID-19 vaccine. Twenty-two percent of White parents shared the same sentiment. Only 10 percent of Hispanic parents who joined the survey declined to vaccinate their children.
It also that 58 percent of parents themselves who did not plan to get the vaccine at all had the same intentions for their children. The same was observed in 63 percent of parents who took a “wait and see” approach themselves.
Respondents who participated in the survey cited four main factors for their hesitancy regarding vaccination. These included possible short-term side effects, unknown long-term side effects, lack of opportunity for long-term studies and expedited vaccine development.
Cincinatti Children’s Hospital pediatrician Dr. Mary Carol Burkhardt said the hesitation is natural as people are naturally cautious with their children. “We’re certainly seeing both sides of the coin. Some parents want to be first in line and want to get their kids protected. [On] the other side, we have a lot of families who are not hesitant but don’t want to be first,” she remarked. (Related: US health experts warn about giving coronavirus jabs to children.)
Pfizer anticipates a full approval from the FDA to help make mandatory vaccination a reality
Meanwhile, the New York Times reported that drug manufacturer Pfizer has applied for an EUA for its vaccine for children aged two to 11 years old. Current authorizations for Pfizer’s two-dose jab issued by the Food and Drug Administration (FDA) only permit its use on individuals 12 years old and above.
During a May 11 earnings call, the company told reports and Wall Street analysts that it plans to submit an application allowing the use of its BNT162b2 vaccine on younger patients. A day before the earnings call, the FDA permitted Pfizer to use its vaccine on children 12 to 15 years old. This effectively amended its earlier authorization from December 2020. (Related: Two-year-old baby DIES during Pfizer’s Covid-19 vaccine experiments on children.)
Pfizer continued that it is applying for a full approval of its vaccine for people aged 16 to 85. Full approval from the FDA will allow Pfizer to directly market the two-dose vaccine to consumers. However, it will take months before the regulator fully approves Pfizer’s vaccine – made in partnership with German company BioNTech.
Aside from the Pfizer vaccine, the Moderna and Janssen vaccines have also been granted emergency approval in the U.S. Vaccines approved under emergency use are only allowed on a temporary basis. Their approvals can be revoked once health authorities declare that a public health emergency is over. Full FDA authorization will permit vaccines to remain on the market even beyond the pandemic’s end.
Many entities such as schools and businesses are anticipating the FDA granting full approval to COVID-19 vaccines in a bid to make vaccination mandatory. Two public universities in California have taken the lead in requiring inoculation for community members. The University of California (UC) and California State University (CSU) systems announced that students, faculty and staff members must get vaccinated against the Wuhan coronavirus once the vaccines receive full approval.
CSU spokeswoman Toni Molle remarked: “We are announcing now so that students and employees have time to receive a vaccination.” She continued that a formal mandate will depend on the FDA granting full authorization on the vaccines and CSU’s discussions with labor unions. Molle roughly estimated that the mandate could come before or after classes begin in the fall.
Meanwhile, UC issued a separate statement saying that students who plan to attend classes on campus in the fall to update their immunization records. Their records must reflect that they received a COVID-19 vaccination or a medical reason exempting them from getting the vaccine. The statement warned that students or staff members who fail to comply will be “barred from in-person access” to campus programs and facilities – including campus housing.
Visit MedicalTyranny.com to read more about Pfizer’s plans to inoculate children and other sectors with its COVID-19 vaccine.
Sources include:
Tagged Under: Big Pharma, children's vaccine, coronavirus vaccine, covid-19 pandemic, Food and Drug Administration, full authorization, mandatory vaccination, Medical Tyranny, Pfizer, vaccine hesitancy, Wuhan coronavirus, young children
RECENT NEWS & ARTICLES
MedicalTyranny.com is a fact-based public education website published by Medical Tyranny Features, LLC.
All content copyright © 2018 by Medical Tyranny Features, LLC.
Contact Us with Tips or Corrections
All trademarks, registered trademarks and servicemarks mentioned on this site are the property of their respective owners.